Corrosion is a chemical process by which metal, stone, or other materials are broken down via the formation of oxides, hydroxides, or sulfides on the surface. Corrosion Doctors estimate that the combined direct and indirect costs of corrosion for the U.S. are $276 Billion per year, with most costs originating from industrial sectors such as drinking water systems, sewage systems, motor vehicles, and defense. [1] While corrosion due to air is certainly a significant contributor, particularly in ferrous materials, perhaps more significant is corrosion due to liquid solutions, which includes medical implants in an extracellular matrix. This is evident in Corrosion Doctors listing of water and sewage systems, and it can be assumed that naval, and other sea-based activities contribute significantly to the defense and motor vehicles reports. Corrosion Doctors also list ways to better manage the negative effect of corrosion: “Preventative strategies include … increasing awareness of corrosion costs, changing the misconception that nothing can be done about corrosion, and improving education on corrosion.” [1]
Metallic biomaterials are the most used material class in implantable devices. Metals offer advantageous properties for many biomedical applications, but the environments in the human body can be considered highly aggressive towards metals. When placing a metal in a solution, corrosion occurs, promoting the forward reaction of (in the case of Fe, Iron) FeFe2++2e-. Thus, the metal is corroded. The reverse reaction is called protection, and in equilibrium, both reactions occur simultaneously. Extending this idea, when two metals are held against each other in a galvanic cell, one will corrode, and one will remain protected. Which metal undergoes which process is dependent on the differing electric potentials of the two half-cell reactions. For example, if the reference electrode being used is an Ag/AgCl electrode: AgCl+e-Ag(s)+Cl- with a fixed potential of 0.197 V. Electrons will naturally pass through a conducting wire, from the lower potential metal (anode) to the higher potential metal (cathode). This galvanic cell process is displayed in Figure 1.
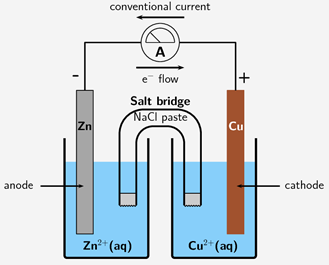
Figure 1. Diagram of Galvanic Cell [2]
Thus, the Zn electrode is corroded, and the Cu electrode is protected. Electrical perturbation methods can be performed to characterize corrosion [3]. Perturbation methods utilized for these measurements include potential-electrochemical impedance spectroscopy (PEIS) and Tafel plot (TP); the latter of which is a form of cyclic voltammetry (CV) perturbation. The TP method varies voltage linearly as a scan between a high and low applied potential difference and measures the change in current, while PEIS oscillates between an applied voltage range between a low and high value as a sinusoidal wave and monitors the current response. Tafel fitting fits the two linear portions on either side of a log|I| vs Ewe plot. PEIS perturbation can calculate the corrosion resistance, however, is incapable of providing the corrosion current. The TP method, however, can calculate the corrosion current. Tafel fitting of the resulting plots yields the corrosion potential in addition to corrosion current. These corrosion performance values give information about the nature of the corrosion including if corrosion occurs or how fast corrosion will occur.
The cost of corrosion amounts to a very large number each year worldwide and causes implications in a variety of industries. Metal bearing systems have an extensive range of biomedical applications, including joint, knee, and hip replacements, splints, plates, screws, braces, and many more. Corrosion is the destructive chemical or electrochemical attack of a material and is a very common daily occurrence in various metal-bearing systems.
Are you struggling to get your device or drug FDA approved? Give EMMA International a call today at 248-997-4497 to learn which regulatory pathway is right for your product. We specialize in full-circle consulting to help get your product approved and out to market in the best way possible.
[1] “Welcome to Corrosion Doctors.” Corrosion Science and Engineering Information Hub, Metallurgical Technologies Inc., corrosion-doctors.org/index.htm
[2] “Galvanic and Electrolytic Cells.” Siyavula Technology-Based Learning, www.siyavula.com/read/science/grade-12/electrochemical-reactions/13-electrochemical-reactions-03.
[3] Society, R. (2017, June 13). Fig. 1 Schematic of the three-electrode measurement system. ResearchGate. https://www.researchgate.net/figure/Schematic-of-the-three-electrode-measurement-system_fig1_317584643





