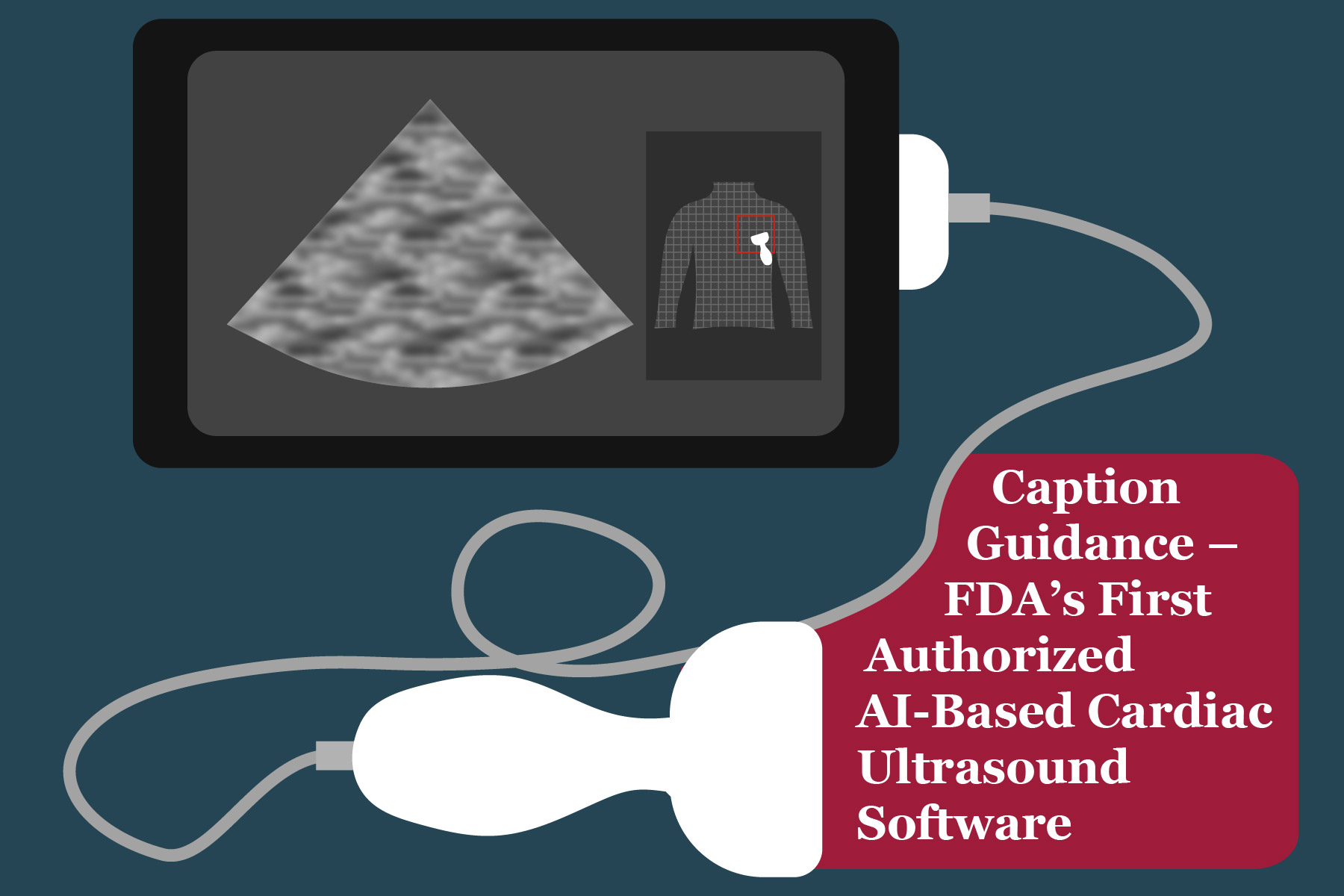
Happy American Heart Association month, everyone! While celebrating this month at EMMA International, we bring you this blog on a medical software tool that utilizes Artificial Intelligence (AI) for providing echocardiography. This is the story of Caption Guidance, the world’s first and only FDA-granted AI-guided cardiac ultrasound software.
Built by the California-based Caption Health, the Caption Guidance tool employs AI algorithms to provide its intended use. The major functionality of this tool is to guide healthcare professionals in getting the best quality images or videos while performing ultrasound or echocardiography. From a software perspective, the principal emphasis of the algorithm is to provide a distinction between images or videos which are acceptable, and which do not have the expected clarity.1
For users, the application provides directions while they are moving the probe on the patient’s chest for cardiac imaging. The application instructs to shift the probe towards an accurately computed direction if the currently generated image or video has noise or is not clear. Also, the images and video frames with the right quality are auto-captured. But in the end, I wondered, how is this image or video quality measured? That is where I found about DCNNs and the ‘learning’. 1
For Caption Guidance, the AI Algorithm used for image or video frame selection is the Deep Convolutional Neural Network (DCNN). DCNNs are the most powerful and preferred AI algorithms when it comes to imaging applications. The learning begins by training this DCNN with a massive data set of existing images and videos. When the user shifts the probe while performing an ultrasound on the patients’ body, new images or videos are generated and these are then compared with the learned data. Based on the minimal difference from image quality comparison, current images and video frames are selected.2
This imaging software tool followed the FDA De Novo pathway, indicating the device fell in the low-to-moderate risk category. Truly, as the selected images and videos assist healthcare professionals to make significant decisions relating to patient care, the software’s reliability, performance, and most important, safety must be thoroughly tested.
Software validation entails testing the software design and application thoroughly for ensuring the software reaches the safety, quality, and efficiency levels expected by the FDA. These include activities such as documenting the software requirements, architecture, performing risk-assessment, and in-depth code testing with inspections. Specifically, with AI-based medical devices, the requirements, training datasets, and AI models may change frequently. To regulate such a software that has a dynamic design pattern, the FDA recently released a detailed action plan that specifies what steps they will be implementing for specifically regulating AI-based medical devices. The action plan includes activities such as gathering data for real-time AI performance monitoring and building the best consensus standards for improving AI models, but the major takeaway is that this year, they will be providing a specific guidance document that will focus on regulating AI/ML-based software as a medical device.3
As discussed, one of the most advanced echocardiography software tools in the healthcare industry is the Caption Guidance. It uses AI to guide novice healthcare professionals to get quality images and videos while performing cardiac imaging or ultrasound. But manufacturers or AI-engineers should ensure that their tool is completely validated and tested before it is hosted in the market. This is where we can help. Do you have an AI/ML-based software tool that needs to be validated or FDA-compliant? Our quality and software experts can get your software tool completely validated and guide you through the FDA regulatory process to ensure your AI/ML-based medical device is FDA compliant. Contact us at 248-987-4497 or email us at info@emmainternational.com for more information.
1FDA (February 2020). FDA Authorizes Marketing of First Cardiac Ultrasound Software That Uses Artificial Intelligence to Guide User. Retrieved on February 9th, 2021 from https://www.fda.gov/news-events/press-announcements/fda-authorizes-marketing-first-cardiac-ultrasound-software-uses-artificial-intelligence-guide-user.
2Schneider, M., Bartko, P., Geller, W. et al. A machine learning algorithm supports ultrasound-naïve novices in the acquisition of diagnostic echocardiography loops and provides accurate estimation of LVEF. Int J Cardiovasc Imaging (2020).
3FDA (January 2021). FDA Releases Artificial Intelligence/Machine Learning Action Plan. Retrieved on February 9th, 2021 from https://www.fda.gov/news-events/press-announcements/fda-releases-artificial-intelligencemachine-learning-action-plan.