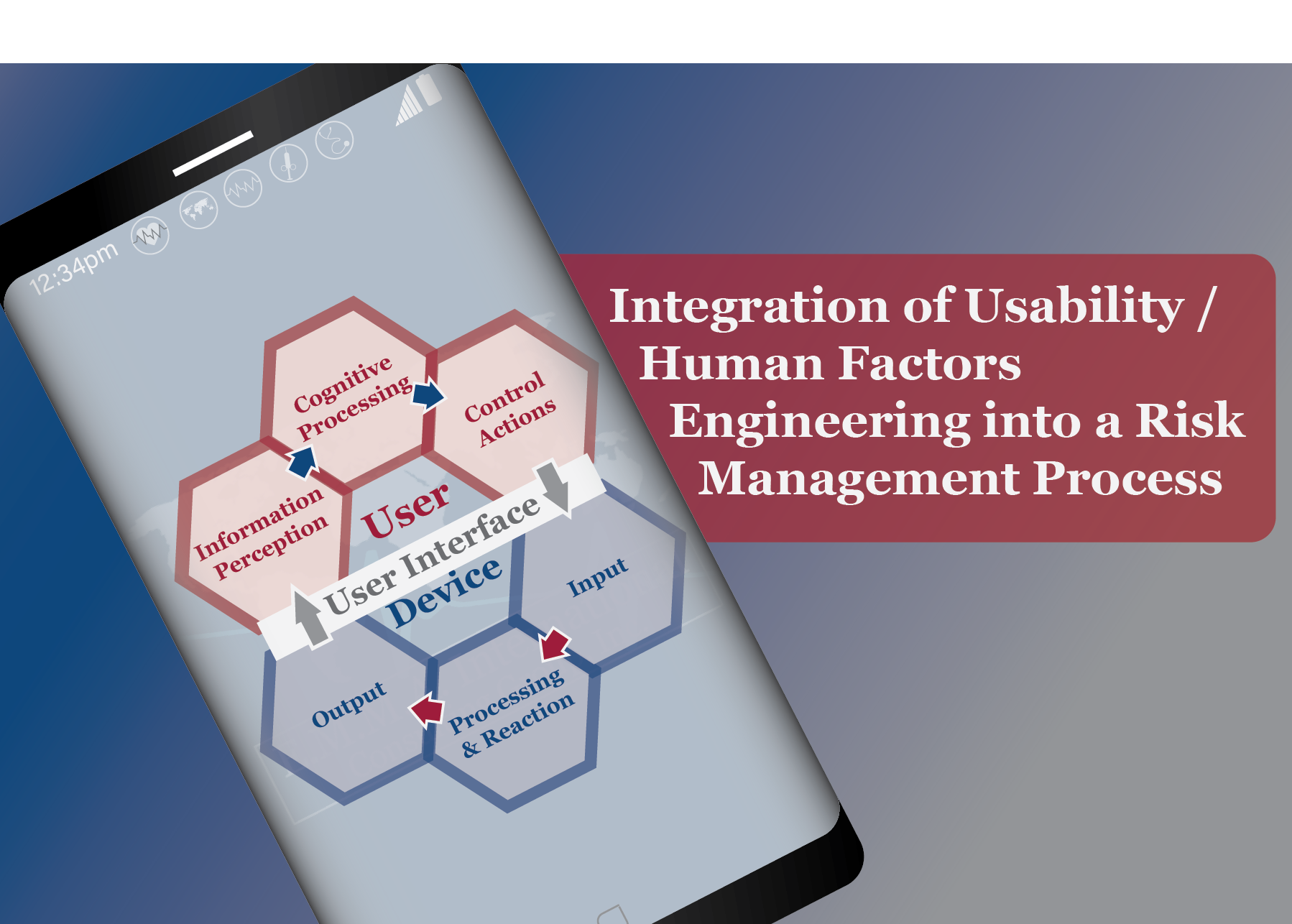
Does your medical device Risk Management process include Usability / Human Factors Engineering (HFE)?
For many devices, the manufacturer must have a process to evaluate the Usability of a medical device as it relates to safety. This can be accomplished by adding processes to, or integration with, existing Quality Systems. Decisions on how and when to integrate Usability into the design and development process can have an impact on an organization’s product development lifecycle.
Usability, or HFE, can be integrated with most medical device Design Control and Risk Management processes which are part of a larger Quality System. Usability considerations in the early phases of the design and development of a device can include:
-Who is the customer (User)?
-Where is the user located?
-What is the Intended Use of the device?
-How does the device interface with Users?
-Risks associated with the use of the device.
These considerations will help a manufacturer begin the process of defining the scope of the Usability / HFE process for their device. Having one or more standards that could apply to a device, it is important to understand the scope of work and compliance strategy to meet all applicable requirements. Standards that support the device design could include, but are not limited to:
- FDA Guidance on Applying Human Factors and Usability Engineering to Medical Devices
- AAMI/ANSI HE75 Human Factors Engineering – Design of Medical Devices
- ANSI/AAMI/IEC 62366 Medical devices – Application of usability engineering to medical devices
- ANSI/AAMI/ISO 14971 Medical Devices – Application of risk management to medical devices
- IEC 60601-1-6: Collateral standard: Usability
- IEC 60601-1-8: Collateral Standard: General requirements, tests, and guidance for alarm systems in medical electrical equipment and medical electrical systems.
- IEC 60601-1-11 Medical electrical equipment: Collateral Standard: Requirements for medical electrical equipment and medical electrical systems used in the home healthcare environment.
A systematic approach for a manufacturer to understand which requirements apply to their device and how to manage those requirements is needed early in the product development process. Subsequent development phases likely include developmental Usability, or Formative, studies. Having the device, Users, Use Cases, Use Environments and risks identified before, and during these studies, are necessary steps for successful subsequent Human Factors Validation or Summative testing.
With a device developed through formative studies being completed and results reviewed, planning for Human Factors Validation testing can begin. A medical device Quality System with Design Controls and Risk Management processes that have been developed for the application of Usability / HFE will allow for validation activities to be completed with minimal issues, resulting in shorter product development timelines and improved customer experiences.
Having trouble building your Risk Management program? Give us a call at 248-987-4497 or email us at info@emmainternational.com.