Navigating the regulatory landscape for dietary supplements is crucial for manufacturers, distributors, and consumers alike. With the growing popularity of vitamins, minerals, herbs, and other health-enhancing products, understanding the requirements that govern these supplements is essential. This blog provides an overview of the key regulatory requirements for dietary supplements in the United States and other major markets.
Regulatory Overview in the United States
In the United States, dietary supplements are regulated by the FDA under the framework established by the Dietary Supplement Health and Education Act (DSHEA) of 1994. This act defines dietary supplements as products taken orally that contain dietary ingredients intended to supplement the diet. These ingredients can include vitamins, minerals, herbs or other botanicals, amino acids, and various other substances.
The manufacturing of dietary supplements must adhere to Current Good Manufacturing Practices (cGMPs), as specified in 21 CFR Part 111. These regulations are designed to ensure that supplements are produced consistently and meet stringent quality standards. This encompasses everything from quality control procedures that ensure the identity, purity, strength, and composition of dietary supplements, to the registration of manufacturing facilities with the FDA, and the meticulous record-keeping of production, packaging, labeling, and distribution processes.
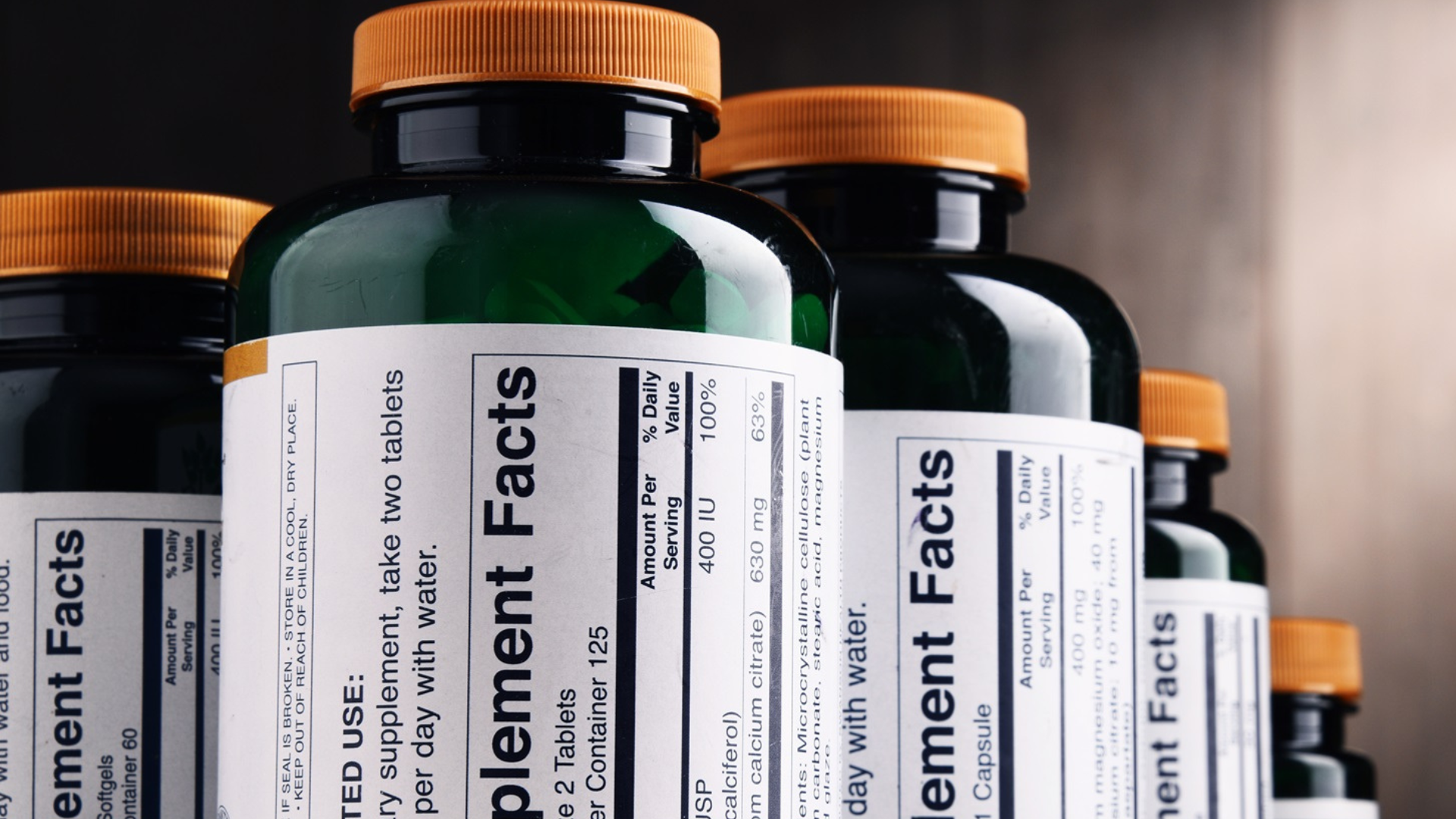
Labeling is another critical component of dietary supplement regulation. The FDA mandates that labels must clearly state that the product is a dietary supplement, include the net quantity of the product, and provide a Supplement Facts panel. This panel details the serving size, ingredients, and the amount of each dietary ingredient per serving. Additionally, labels must offer directions for use and include the name and place of business of the manufacturer, packer, or distributor.
When it comes to claims and marketing, the FDA permits three types of claims on dietary supplements: health claims, nutrient content claims, and structure/function claims. Health claims, which describe the relationship between a dietary supplement ingredient and the reduction of a disease or condition risk, require FDA pre-approval. Nutrient content claims detail the level of a nutrient in the product, such as “high in vitamin C,” while structure/function claims describe how the product may affect the body’s structure or function, like “supports immune health.” These claims must be substantiated and include the disclaimer that the statement has not been evaluated by the FDA and that the product is not intended to diagnose, treat, cure, or prevent any disease.
Unlike pharmaceutical drugs, dietary supplements do not require FDA approval before they are marketed. However, the FDA conducts post-market surveillance to monitor the safety of these products. Manufacturers are obligated to report serious adverse events to the FDA, which has the authority to take action against products found to be adulterated or misbranded.
Regulatory Requirements in Other Markets
The regulatory landscape for dietary supplements varies significantly across different countries. In the European Union, dietary supplements are regulated as foodstuffs under the Food Supplements Directive 2002/46/EC. This regulation ensures that supplements do not pose a health risk and meet specified purity criteria. Labels in the EU must include the name of the nutrient or substance, the amount per serving, the recommended daily intake, and a warning not to exceed the stated dose.
In Canada, dietary supplements are classified as Natural Health Products (NHPs) and are regulated under the Natural Health Products Regulations by Health Canada. Before being sold, NHPs must be licensed, and the license number (NPN) must appear on the label. Canadian regulations also require manufacturers to follow strict production standards similar to cGMPs in the U.S. and mandate that labels provide accurate information, including recommended use, active ingredients, and warnings.
Challenges and Considerations
Compliance with regulatory requirements is essential but can be challenging due to the variation in regulations across different countries, the evolving nature of regulatory frameworks, and the need to substantiate all claims. Companies operating in multiple markets must navigate these complexities to ensure their products are safe, effective, and compliant with local laws. By doing so, they can build consumer trust and ensure the longevity of their brand in a competitive and highly regulated industry.
Understanding and adhering to these regulatory requirements is crucial for success in the dietary supplement market. Whether operating in the United States, Europe, Canada, or beyond, manufacturers and distributors must ensure that their products meet all legal standards. This not only protects consumers but also enhances the credibility and sustainability of the companies in this rapidly growing industry.
EMMA International specializes in providing comprehensive regulatory and compliance support tailored to the unique needs of dietary supplements manufacturers. EMMA International offers in-depth knowledge of FDA regulations, including Current Good Manufacturing Practices (cGMP), New Dietary Ingredient (NDI) notifications, and labeling requirements. Call us at 248-987-4497 or email info@emmainternational.com to learn more.
FDA (Feb 2024) Dietary Supplements retrieved from: https://www.fda.gov/food/dietary-supplements