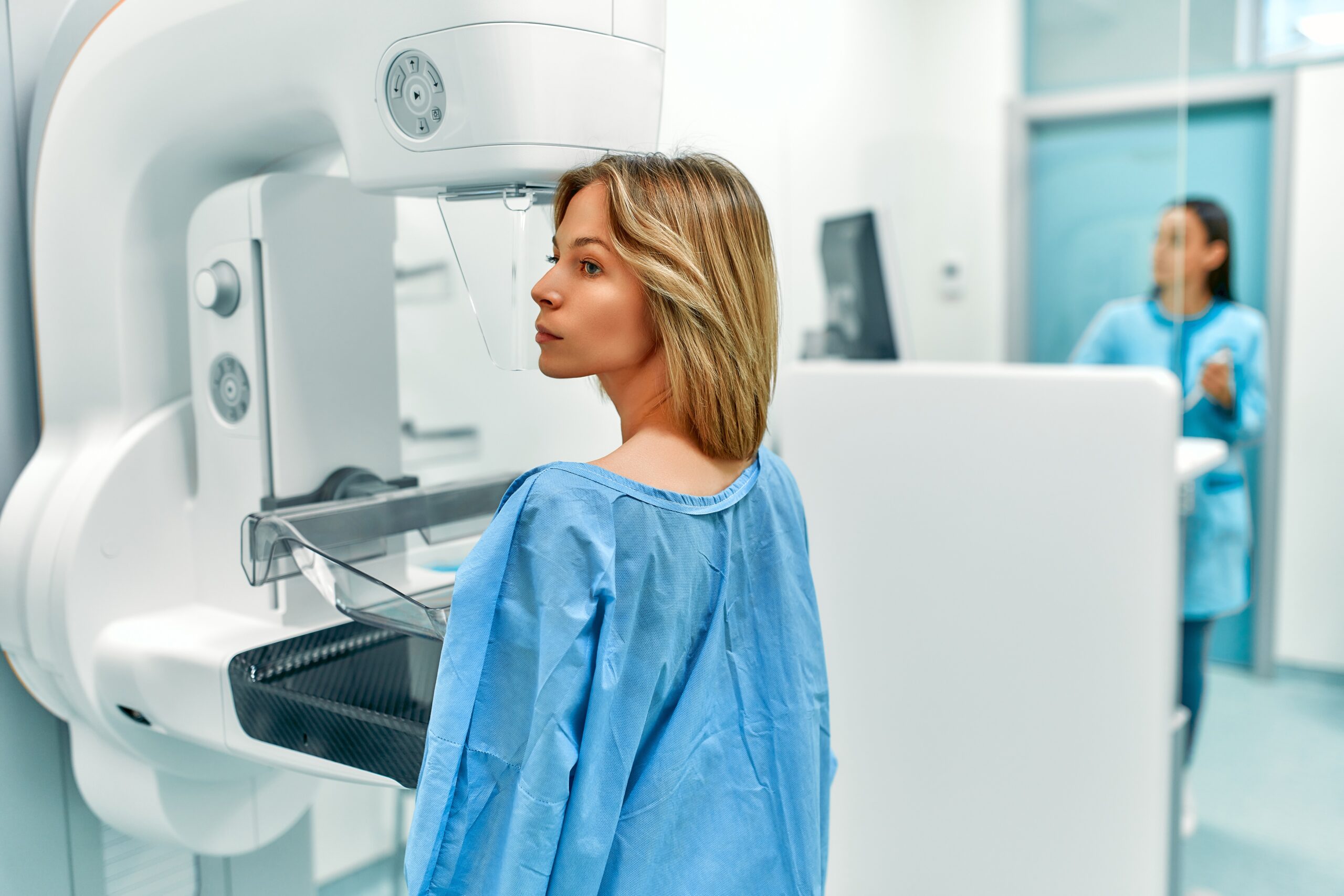
Mammograms play a vital role in the early detection of breast cancer, helping to reduce mortality rates by identifying abnormalities in breast tissue before they can be felt. To ensure the safety, efficacy, and quality of mammography technology, the FDA maintains stringent regulations over mammogram machines and facilities. In this blog, we’ll explore how mammogram technology works and how the FDA regulates it to ensure patient safety.
The Technology Behind Mammograms
Mammograms use low-dose X-rays to capture images of the breast tissue. There are three main types of mammography technology:
- Film Mammography (Conventional) In this traditional method, X-ray images are captured directly onto film. While effective, film mammography has largely been replaced by digital mammography due to its limitations in image storage and manipulation.
- Digital Mammography Also known as full-field digital mammography (FFDM), this modern technology captures images electronically, allowing for greater flexibility in image enhancement, manipulation, and storage. Digital mammograms also expose patients to lower doses of radiation compared to film mammograms.
- 3D Mammography (Digital Breast Tomosynthesis) This newer technology takes multiple images of the breast from different angles, producing a 3D image. This can be particularly useful in detecting cancers in dense breast tissue, as it reduces the overlap of tissues seen in traditional 2D mammograms.
How the FDA Regulates Mammograms
Mammography, being a critical diagnostic tool, falls under the purview of the FDA. The FDA regulates mammogram technology and facilities through several key initiatives:
- Mammography Quality Standards Act (MQSA) The MQSA, enacted by Congress in 1992, is the primary legislation that governs mammography facilities in the United States. Under this act, all mammography facilities must meet strict standards related to equipment, personnel, and quality control to ensure the reliability of mammograms. The act covers:
- Accreditation: All mammography machines must be accredited by an FDA-approved body, ensuring that they meet set performance standards.
- Certification: Every facility that offers mammography must be certified by the FDA or an approved state regulatory agency.
- Inspections: Facilities must undergo annual inspections to verify adherence to quality standards in areas like image quality, radiation dose, and technician qualifications.
- Personnel: Radiologists, technologists, and medical physicists working with mammograms must meet specific educational and training requirements.
- Device Approval Process Mammography machines are classified as Class II medical devices by the FDA. Before a new mammogram technology can be used in clinics, it must go through the FDA’s 510(k) clearance process. This ensures that the new device is substantially equivalent to a previously cleared device (predicate device) in terms of safety and effectiveness. For more advanced technologies, like 3D mammography, the FDA may require additional clinical data to prove their benefits.
- Radiation Safety The FDA, in collaboration with other organizations like the American College of Radiology (ACR), ensures that mammogram machines meet radiation safety standards. These standards regulate the amount of radiation used during imaging to minimize patient exposure while maintaining diagnostic image quality.
Advancements in Mammography and Future FDA Regulations
As technology evolves, the FDA continues to adapt its regulatory framework to ensure that new developments in mammography—like artificial intelligence (AI) and machine learning (ML) tools used for image analysis—are safe and effective. AI tools, for example, are being explored for their potential to assist radiologists in detecting subtle abnormalities that may be difficult for the human eye to catch. As these technologies emerge, the FDA will play a key role in evaluating their clinical performance and ensuring they meet the highest safety and quality standards.
Mammography remains a cornerstone of breast cancer screening, with evolving technologies like digital and 3D mammography improving diagnostic accuracy and patient outcomes. Through the Mammography Quality Standards Act and device regulation, the FDA ensures that both the technology and the facilities providing mammograms operate at the highest levels of safety and effectiveness.
As a woman-owned business, EMMA International is deeply committed to improving women’s health through innovative solutions and regulatory expertise. We understand the unique challenges and opportunities within the women’s health space, and we are passionate about supporting life sciences organizations in developing and delivering products that make a meaningful impact on women’s lives. Call us at 248-987-4497 or email info@emmainternational.com to learn more.
FDA (Sep 2024) Mammography Quality Standards Act (MQSA) and MQSA Program retrieved from: https://www.fda.gov/radiation-emitting-products/mammography-quality-standards-act-mqsa-and-mqsa-program