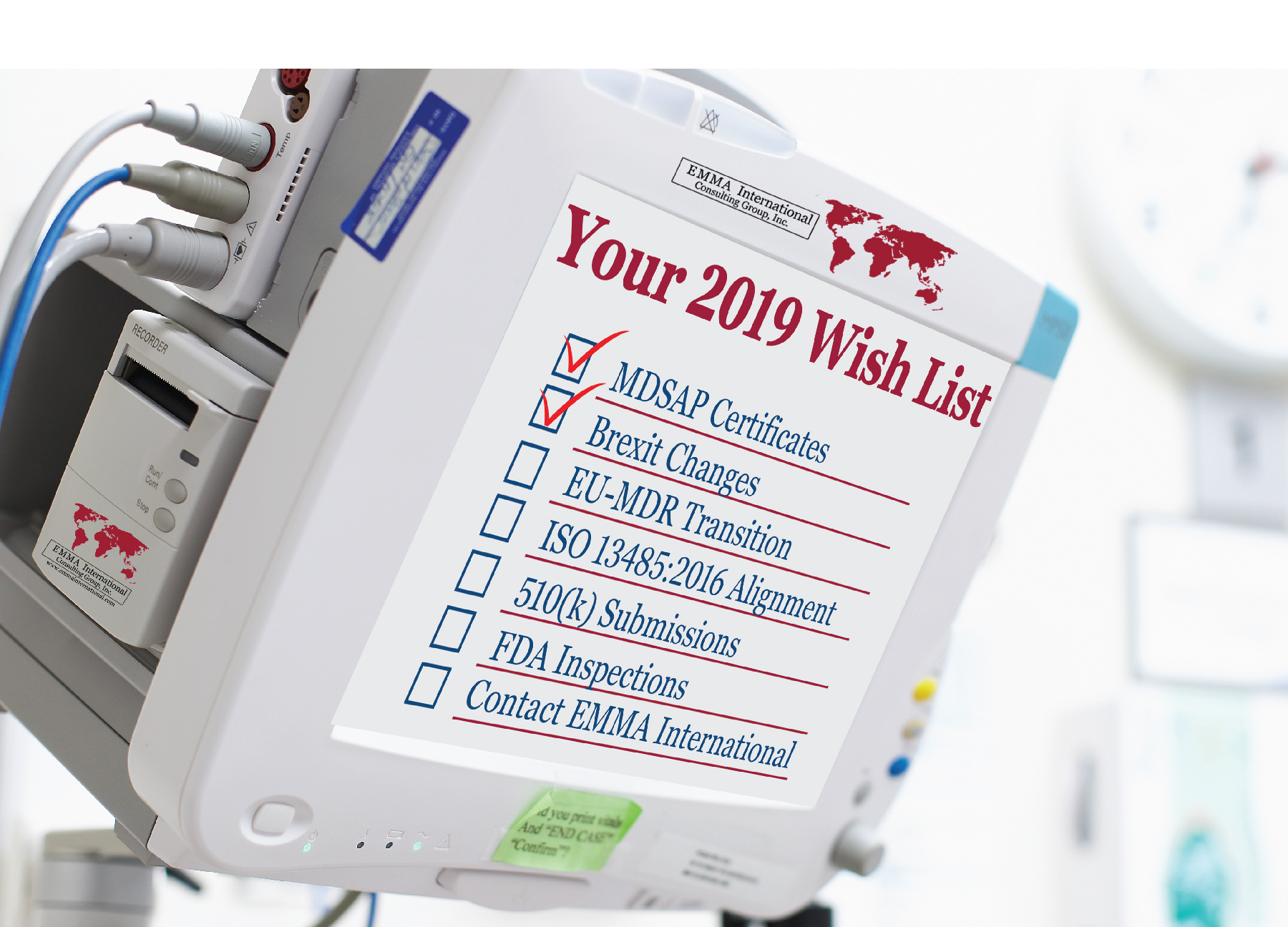
We witnessed several changes in the medical device industry in the year 2018. The impact of some changes was hard-hitting while some were received with a warm welcome by industry professionals. It sure is a difficult and confusing time for the medical device industry to adopt these new changes, while still being able to maintain the financial stability to incorporate the new global regulations.
We here at EMMA International thought of putting together a quick list to help you plan your compliance goals for the year 2019. Whatever your needs are, EMMA International has the expertise to help you all the way.
- Thinking about marketing your device in Canada? You will need your MDSAP certificate in place first. The transition period for device makers to obtain MDSAP certificates has officially ended on December 31st, 2018 and on January 1, 2019, Health Canada will only allow MDSAP certified companies to legally market their devices in Canada.
- The biggest event in 2019 for the medical device industry throughout the world is the Brexit. Britain is set to exit from the European Union on March 29, 2019. No deal has been struck between the two parties yet. We mentioned in our blog “A NO-DEAL BREXIT AND THE IMPACT ON MEDICAL DEVICES” a few weeks ago the consequences that the medical device industry will have to face in the case of a No-deal Brexit. A quick recap of some measures that you can take until any decisions are finalized:
- Appoint an authorized representative (AR) in the EU if your current AR is based in the UK
- If you currently hold a CE mark from a notified body based in the UK, you might have to transfer the certificate to an EU-based NB to continue marketing your device in Europe
- We are currently in the transition period to comply with the EU-MDR, which is set to get into force in May 2020. So, if you haven’t started transitioning into the new EU-MDR or are stuck in the process and need help, EMMA International can help you succeed and obtain your goals and objectives.
- A huge announcement was made by the FDA commissioner, Dr. Gottlieb, to align the FDA’s QSR [21 CFR Part 820] with ISO 13485:2016 in FDA’s Fall unified agenda. The impact of which we discussed in our blog “FDA TO ALIGN WITH ISO 13485:2016”. The decisions are to be finalized in the Spring of 2019. However, to be ahead of the game, you can start preparing by conducting a gap analysis of your quality system to comply with ISO 13485:2016 and avoid any last-minute disruptions. Of course, EMMA International can do this and all the other heavy lifting for you, so you are ready for this transition with a world class QMS.
- Planning to file a 510(k) for your new device? A statement released by the FDA1
in November 2018 states that all the new 510 (k) submissions will now have to prove substantial equivalence to a device that is not more than 10 years old. Thus, if you have already decided on a predicate for your new device that is more than 10 years old, you might have to reconsider it before filing your 510(k).
- The FDA has once again proved that it has taken up a new approach of conducting more inspections to ensure that companies stay in compliance with the QSR, rather than undertaking enforcement actions. It is always a good idea to be prepared at all times for an FDA inspection. Having said that, we can help prepare you and your team with our pre-audit tool kit and engage in a remediation plan to achieve the desired state of compliance. If you have received a 483 or a warning letter already, then give us a call right away and we will advocate for you.
Whatever your business goals are, be rest assured that all your compliance needs will be taken care of by EMMA International. Start the new year off right by calling us and accomplishing all your new year goals. You can reach us at 248-987-4497 or info@emmainternational.com.
1FDA (November 2018) Statement from FDA Commissioner Scott Gottlieb, M.D. and Jeff Shuren, M.D., Director of the Center for Devices and Radiological Health, on transformative new steps to modernize FDA’s 510(k) program to advance the review of the safety and effectiveness of medical devices retrieved on 01-02-2019 from https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm626572.htm