Our clients have extensive experience and familiarity with CLIC, but for many of you out there, it is a new concept. Thus, I decided to write a multipart blog series discussing CLIC as a concept and how it can help your organization achieve the required level of compliance.
Our QMS as a Self-Learning System – a different perspective
At the heart of a QMS is the rule-based Core, a multi-layered set of Procedures and Data Structures (forms) that define the architecture and stipulate the operation, interoperation, and behavior of an organization to achieve consistent high value results.
In most implementations of a QMS, the CAPA process initiates changes to the Procedures to effect updates, corrections or improvements that address the difference between the actual or predicted performance of the QMS vs. the desired ideal performance.
The QMS can thus be rationalized into a simple operational block diagram; the procedural rule- based component represents the output and actuator of the system – the component that controls the business operation. The CAPA function is considered the instigator of changes to the procedural core based on various input events.
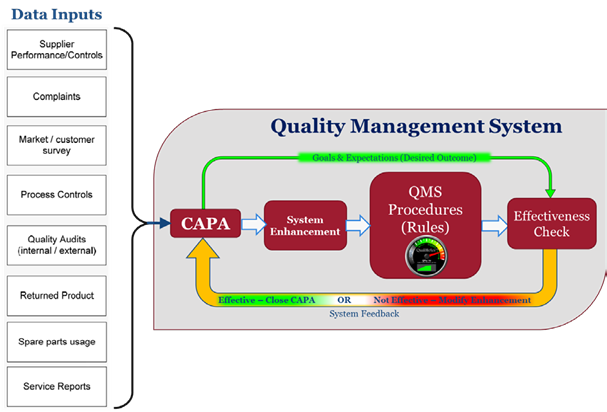
It becomes apparent that this is a closed loop system (i.e., a system that calculates the difference, gap or error between the desired outcomes and the actual outcomes and returns the result for evaluation as effective or non-effective). For any given input, it may take just one loop or perhaps more iterations through these steps before the desired system performance/outcome is achieved; conceptually each of these iterations is a step towards (convergence on) the desired optimal outcome.
The Procedural core of this system is quite unlike a typical static control algorithm in that it is adaptive and is modified on an iterative basis to improve its performance. This is actually the basis for a Self-Learning system or a Neural Network1.
If we now consider a business organization as a competitive entity in a market with numerous competitors, then apply the principles of Game Theory2 for Strategic advantage, we could conclude that advancing the learning and thus optimization of the QMS faster than the competitive field could lead to superior business success from higher quality, more efficient operations at a reduced risk.
In an ideal world, businesses would be able to measure the speed of their QMS self-learning towards the ideal optimized state, the Optimization Velocity. We would then expect to see a significantly higher Optimization Velocity (at lower cost) for a business with CLIC vs. a standard QMS as a result of significantly improved efficiencies in initiating and processing CAPAs within the integrated QMS environment.
If you’d like to learn more about CLIC, feel free to reach out to me directly. Also, if you have any quality, regulatory, or staff augmentation needs, then EMMA is the right service provider for you. We are better than the competition because we provide our clients with a true consulting experience. We can be reached at 248-987-4497, or info@emmainternational.com.
1 Michael A. Nielsen, “Neural Networks and Deep Learning”, Determination Press, 2015, http://neuralnetworksanddeeplearning.com/chap1.html#perceptrons
2 Financial Post: “Using Game Theory to Improve Strategic Decision Making”, Mitchell Osak, July 24, 2010, https://business.financialpost.com/executive/using-game-theory-to-improve-strategic-decision-making





