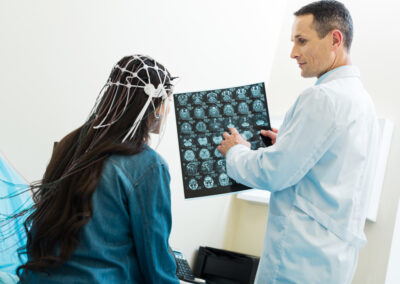
Medical devices embedded with magnets play a pivotal role in modern healthcare, offering innovative solutions for ...
The history of HIV therapies in the United States is a testament to scientific ingenuity, resilience, and the ...
The pharmaceutical industry is undergoing a transformative shift, embracing innovative methods to enhance the ...
This blog explores the history of clinical trial regulations in the U.S. and highlights the pivotal moments that ...
This blog explores the unique cybersecurity challenges faced by the healthcare industry and the pivotal role of ...
This blog explores the historical milestones in women’s health devices and therapies, focusing on regulatory ...
The FDA’s Total Product Life Cycle (TPLC) program is a comprehensive framework designed to ensure the safety and ...
One of the most important tools in the FDA's regulatory arsenal is the black box warning, also known as a boxed ...
When issues arise, remediation projects become essential to address non-compliance, rectify defects, and bring ...
This legal principal mandates that courts defer to administrative agencies' interpretation of ambiguous statutory ...
Bingham Farms, MI, July 12, 2024 –E.M.M.A. International Consulting Group, Inc. (EMMA International), a global ...
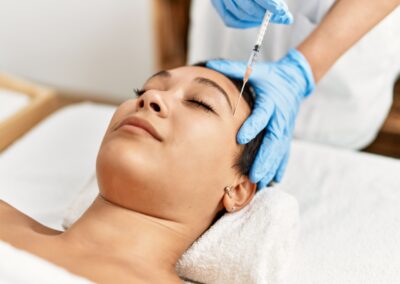
In this blog, we'll explore how the FDA regulates medical aesthetic services to ensure they are safe and effective ...