When issues occur that have the potential to impact product quality, Quality Assurance Reports (QAR) are generated ...
Evolution is the process of how a species changes over time in response to their environment. Often termed ...
The FDA has recently been putting a much greater emphasis on working smarter not harder when conducting clinical ...
Maintenance of equipment is always essential to any manufacturing process. Breakdowns can wreak havoc on any ...
Prior to drugs being commercially available, manufacturers often conduct pre-clinical studies to obtain data ...
While having robust compliance policies and procedures in place is essential for meeting regulatory requirements, ...
One of the biggest and most important aspects of quality management is a system to monitor changes and how they ...
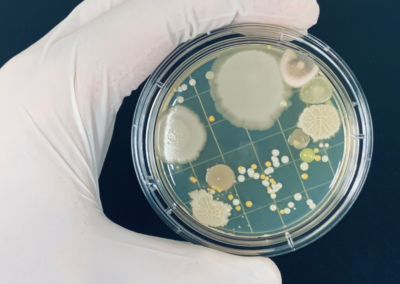
One of the most common techniques to prevent contamination of products and reduce quality impacts in the ...
Having high quality standards is vital to any organization but is even more essential in the pharmaceutical ...
Across all industries, documentation is a key aspect of maintaining good quality and traceability over products. ...
A 510(k) is a premarket submission made to the Food and Drug Administration (FDA) by medical device manufacturers ...
Whether looking to update an existing process or implement something brand new, process design is a critical ...