Nanotechnology is the science of understanding, inventing, and controlling objects which are built in the scales ...
No matter what your product is, at some point in your regulatory journey you will need to appropriately determine ...
If your medical device has contact with human tissue, it is a safe bet that you will be required to conduct ...
Health Canada released a notice for the stakeholders of the medical device and drug industry to help them ...
Biosimilars are biological products that are composed or produced in living systems such as yeast or bacteria. ...
December is HIV/AIDS Awareness Month, and given the state of our current global pandemic, now is a great time to ...
The FDA published 21 CFR Part 11 in 1997 to regulate electronic records and electronic signatures. Technology has ...
Cloud Computing is speeding up the way we create, share, and view data. With features such as multiple backups, ...
If you have ever wondered exactly what role the FDA has in mammography and breast cancer screening, you are not ...
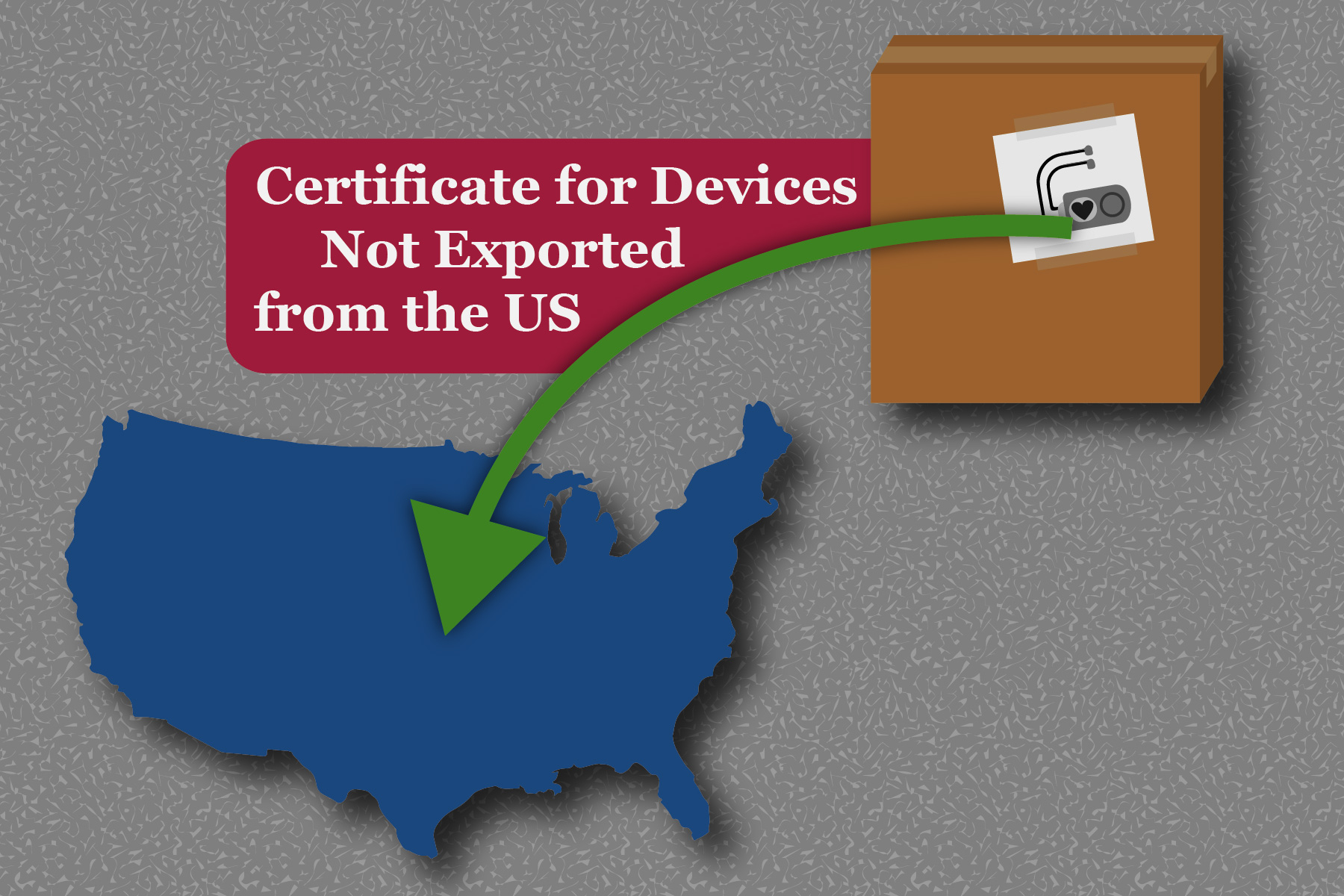
The US FDA introduced a new form of certificate referred to as the Certificate for Devices Not Exported from the ...
Many people had to transition to a remote work environment amidst the COVID-19 pandemic, with Notified Bodies ...
The EU MDR and the IVDR layout the performance and safety requirements for medical devices and In-vitro ...