This legal principal mandates that courts defer to administrative agencies' interpretation of ambiguous statutory ...
Bingham Farms, MI, July 12, 2024 –E.M.M.A. International Consulting Group, Inc. (EMMA International), a global ...
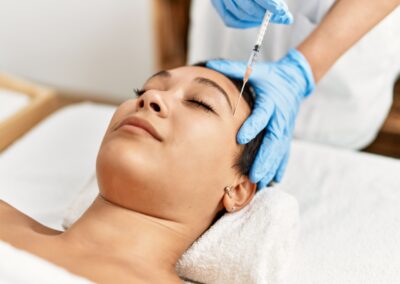
In this blog, we'll explore how the FDA regulates medical aesthetic services to ensure they are safe and effective ...
This blog will explore some of the most groundbreaking medical device advancements that are revolutionizing the ...
This blog discusses the regulatory landscape governing DME distributors across the United States.
One of the most effective ways to achieve and maintain compliance is by performing a regulatory gap analysis. This ...
In the United States, the practice of compounding is regulated under sections 503A and 503B of the Federal Food, ...
In today's globalized and interconnected world, diversity and inclusion are no longer mere buzzwords—they are ...
This blog discusses the importance of FDA's Diversity Action Plans, their impact on clinical research, and the ...
In the realm of pharmaceuticals, blockbuster drugs like painkillers and antibiotics often dominate headlines and ...
One of the notable initiatives is the publication of the Off-Patent, Off-Exclusivity (OPOE) Drug List. This list ...
In this blog, we will explore what HPAPIs are, their applications, benefits, challenges, and the stringent ...