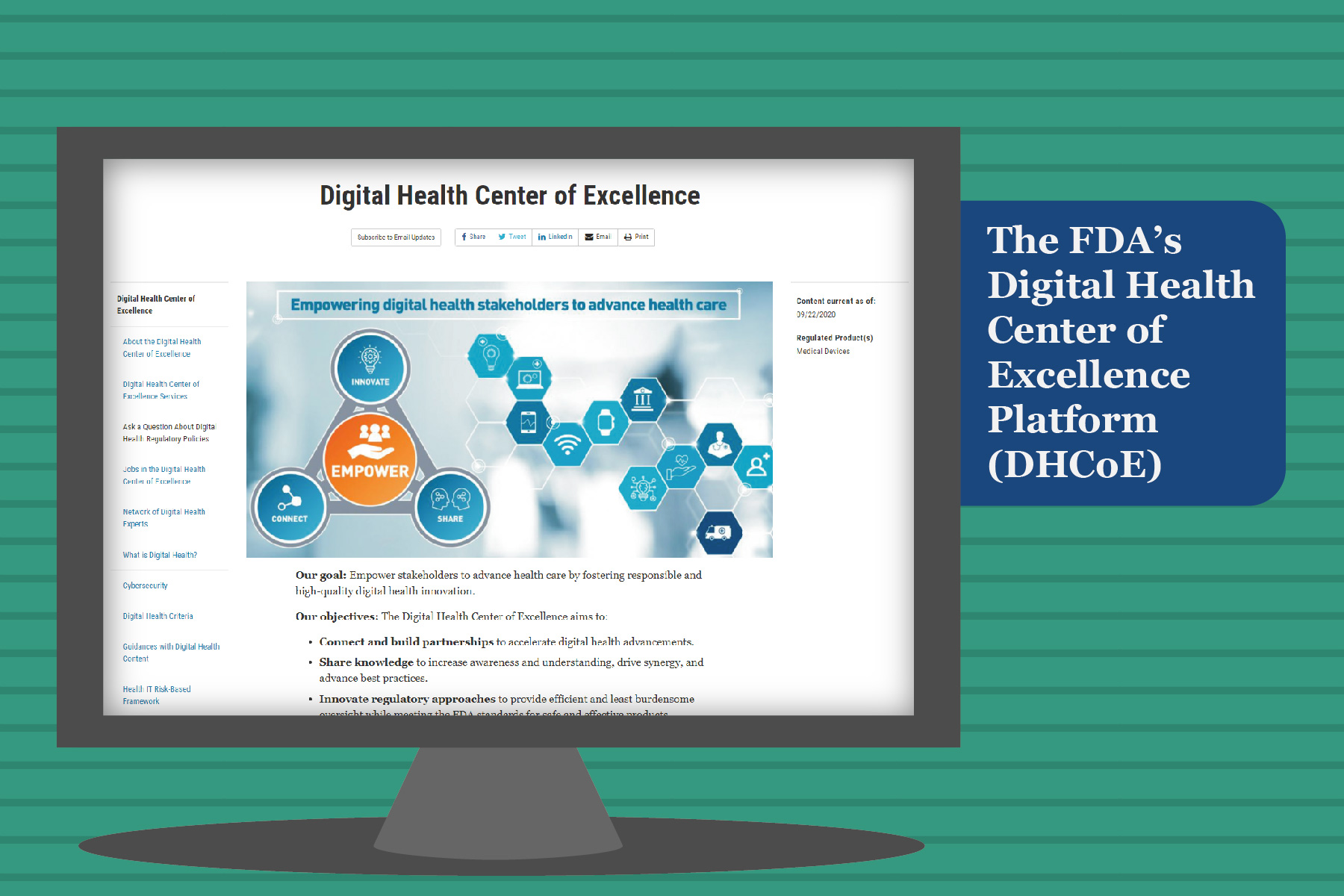
Imagine a platform where innovations meet modern healthcare services while following regulatory requirements. Such ...
FDA recently released a proposed rule that amends its existing regulations around establishing the intended use of ...
One of the major pillars of the current Industry 4.0 is Automation. Indeed, technology is intervening in almost ...
Programmable Logic Controllers (PLCs) are the components that are used for interfacing between multiple hardware ...
Medical device manufacturers are responsible for not only developing safe and effective devices, but also ensuring ...
Data is the single most vital entity of any system and therefore, data storage and management become some of the ...
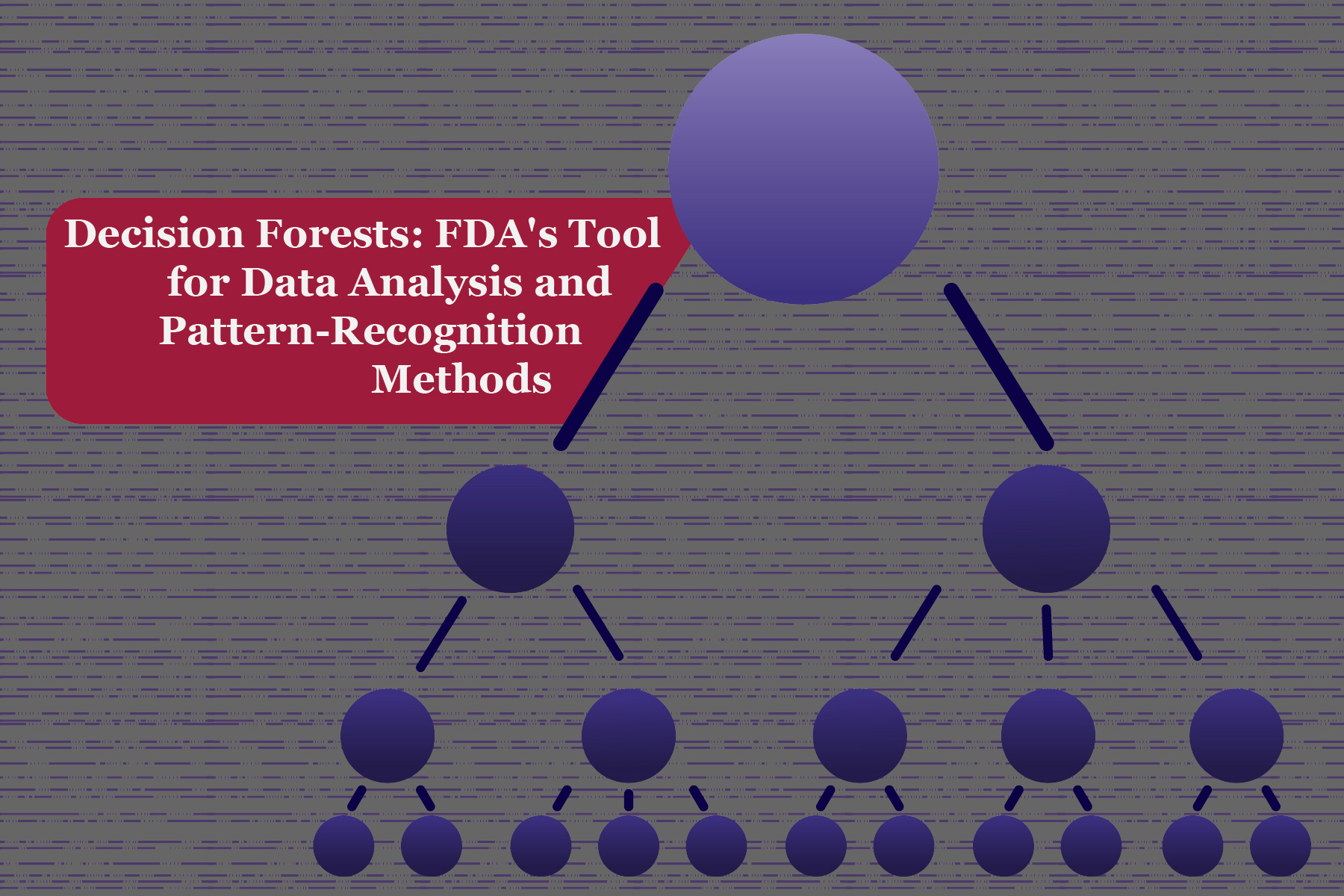
Decision Forests (DFs) are one of the most aggressive data classifying AI algorithms. A decision forest comprises ...
Every medical device company should be familiar with the terms design verification and design validation. You will ...
Health Canada released a new notification on requirements for companies intending to manufacture face shields ...
Approximately 463 million people on Earth are affected by Diabetes, a disease in which the body’s ability to ...
On Monday, the FDA provided an update on its software precertification program known commonly as the Pre-Cert ...
We are deeply saddened by the loss of such a great legal icon and scholar. Justice Ruth Bader ...